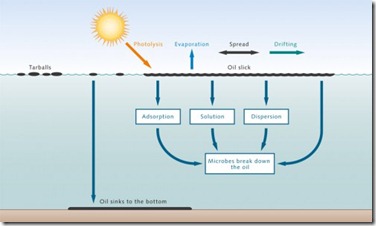
SEWAGE POLLUTION :
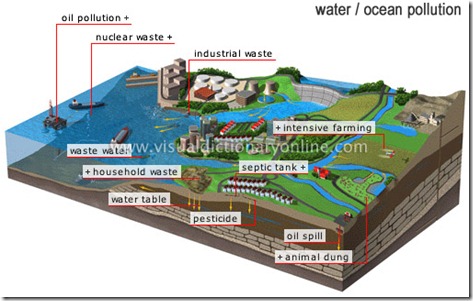
Coastal waters receive a variety of land-based water pollutants, ranging from petroleum wastes to pesticides to excess sediments. Marine waters also receive wastes directly from offshore activities, such as ocean-based dumping (e.g., from ships and offshore oil and gas operations).
One pollutant in the ocean is sewage. Human sewage largely consists of excrement from toilet-flushing; wastewater from bathing, laundry, and dishwashing; and animal and vegetable matter from food preparation that is disposed through an in-sink garbage disposal. Because coasts are densely populated, the amount of sewage reaching seas and oceans is of particular concern because some substances it contains can harm ecosystems and pose a significant public health threat. In addition to the nutrients which can cause overenrichment of receiving waterbodies, sewage carries an array of potentially disease-causing microbes known as pathogens.
Animal wastes from feedlots and other agricultural operations (e.g., manure-spreading on cropland) pose concerns similar to those of human wastes by virtue of their microbial composition. Just as inland rivers, lakes, and groundwater can be contaminated by pathogenic microbes, so can coastal waters. Runoff from agricultural areas also contains nutrients such as phosphorus and nitrogen, which can cause overenrichment in coastal regions that ultimately receive the runoff.
The major types of ocean pollutants from industrial sources can be generally categorized as petroleum, hazardous, thermal, and radioactive. Petroleum products are oil and oil-derived chemicals used for fuel, manufacturing, plastics-making, and many other purposes. Hazardous wastes are chemicals that are toxic (poisonous at certain levels), reactive (capable of producing explosive gases), corrosive (able to corrode steel), or ignitable (flammable). Thermal wastes are heated wastewaters, typically from power plants and factories, where water is used for cooling purposes. Radioactive wastes contain chemical elements having an unstable nucleus that will spontaneously decay with the concurrent emission of ionizing radiation.
Sewage and Agricultural Wastes
Sewage originates primarily from domestic, commercial, and industrial sources. In many developed countries, these wastes typically are delivered either to on-site septic systems or to centralized sewage treatment facilities. In both methods, sewage is treated before being discharged, either underground (in the case of septic tanks) or to receiving surface-water bodies (in the case of sewage treatment plants), typically a stream, river, or coastal outlet.
Although sewage treatment facilities are designed to accommodate and treat sewage from their service area, partly treated or even untreated sewage sometimes is discharged. Causative factors include decayed infrastructure ; facility malfunctions; or heavy rainfall events which overwhelm systems using combined sewers and stormwater drains (known as combined sewer overflows). In unsewered areas, improperly designed or malfunctioning septic tanks can contaminate groundwater and surface water, including coastal waters. In some developed regions (e.g., Halifax Harbor in Nova Scotia, Canada), raw sewage continues to pour into harbors, bays, and coastal waters. In developing countries with no on-site or centralized sanitation facilities, no opportunity exists for any type of treatment, and human wastes go directly into surface waters, including the coastal ocean.
Sewage Sludge
Another source of ocean pollution by sewage-related waste is the disposal of bio solids, a semisolid byproduct of the sewage treatment process, often called sludge. Historically, sludge in developed nations was disposed in coastal waters: New York's twenty sewage treatment plants, for example, once disposed their sludge offshore in a region known as the New York Bight. Although today's environmental regulations in the United States prohibit this practice, sewage sludge is still disposed at sea in some countries.
Disease-causing microbes are the primary human health risk in sewage-contaminated waters, and the main cause of recreational beach closures. Here a sign warns San Diego beachgoers of sewage in the waters.
Agricultural Wastes
Animal wastes often reach water bodies via runoff across the land surface, or by seepage through the surface soil layers. Hence, agricultural runoff containing animal wastes does not receive any "treatment" except what is naturally afforded by microbial activity during its transit to a water body. In coastal watersheds, these wastes can flow through river networks that eventually empty into the sea.
Coastal Eutrophication
Nutrients and organic materials from plants, animals, and humans that enter coastal waters, either directly or indirectly, can stimulate a biological, chemical, and physical progression known as eutrophication. Coastal eutrophication is commonly observed in estuaries , bays, and marginal seas. In a broad sense, coastal eutrophication mirrors the eutrophication of lakes. For example, as increased nutrients stimulate algal and other plant growth, light transmission decreases. The eventual bacterial decay of algae and other plants lowers the dissolved oxygen level in the water. In extreme cases, all of the oxygen can be removed.
Human-accelerated eutrophication (known as cultural eutrophication) can be triggered by inputs of sewage, sludge, fertilizers, or other wastes containing nutrients such as nitrogen and phosphorus. As recently as the 1980s, for example, the New York Bight was essentially lifeless due to oxygen depletion, caused largely by decades of sewage and sludge disposal. As of 2002, Halifax Harbor was still receiving a daily influx of raw sewage, creating serious ecological and public health concerns.
Nutrient-enriched runoff from agricultural land in the midwestern United States is the primary cause of the well-known Gulf of Mexico "Dead Zone." Half of the U.S. farms are located in the Mississippi River Basin, whose entire drainage basin empties into the gulf. Much of the nitrogen reaching the gulf is from agricultural fertilizers, with lesser amounts from residential fertilizers and other sources. The water of the 20,000-kilometer (7,728-square-mile) Dead Zone, extending from the mouth of the Mississippi River Basin to beyond the Texas border, has so little oxygen that essentially no marine life exists.
If human-accelerated eutrophication is not reversed, the entire coastal ecosystem ultimately may be changed. Sensitive species may be replaced by more tolerant and resilient species, and biologically diverse communities may be replaced by less diverse ones. Further, nutrient enrichment and the associated eutrophication in coastal waters is implicated in some harmful algal blooms, in which certain species of algae produce biotoxins (natural poisons) that can be transferred through the food web, potentially harming higher-order consumers such as marine mammals and humans.
Human Health
Sewage, particularly if partially treated or untreated, brings high microbe concentrations into the ocean. Human diseases can be caused by waterborne pathogens that contact the skin or eyes; waterborne pathogens that are accidentally ingested when water is swallowed; or foodborne pathogens found in the tissues of fish and shellfish consumed as seafood. *
Beach pollution consequently is a persistent public health problem. Annually, thousands of swimming advisories and beach closings are experienced because high levels of disease-causing microbes are found in the water. Sewage often is responsible for the harmful microbial levels.
Seafood contaminated by sewage-related pathogens sickens untold numbers of people worldwide. Regulatory agencies will close a fishery when contamination is detected. However, many countries lack regulatory oversight or the resources to adequately monitor their fisheries.
Industrial Wastes
Industrial wastes primarily enter coastal waters from terrestrial (land-based) activities. Industries, like municipalities and other entities that generate wastes, dispose of many liquid wastes through wastewater systems (and ultimately to waterbodies), whereas they dispose of their solid wastes in landfills.
The quantity and characteristics of industrial wastewater depends on the type of industry, its water and wastewater management, and its type of waste pretreatment (if any) before delivery to a wastewater (sewage) treatment plant. Because industrial waste frequently goes down the same sewers as domestic and commercial nonindustrial waste, sewage often contains high levels of industrial chemicals and heavy metals (e.g., lead, mercury, cadmium, and arsenic).
Substances that are not removed by wastewater treatment processes are discharged via the treated effluent to a receiving stream, river, or coastal outlet. Inland waters ultimately reach the ocean, carrying with them some residual chemical that are not attenuated, stored, or degraded during their journey through the watershed. Other land-based sources of industrial pollutants in the ocean are pipeline discharges and transportation accidents, leaking underground storage tanks, and activities at ports and harbors. Intentional, illegal dumping in inland watersheds and in inland waterbodies also can deliver industrial wastes to drainageways, and ultimately to the ocean.
In coastal watersheds, some industries discharge their wastes directly to the ocean. Like industries located inland, these industries must first obtain a permit under the Clean Water Act. Industrial pollutants also can directly enter the ocean by accidental spills or intentional dumping at sea.
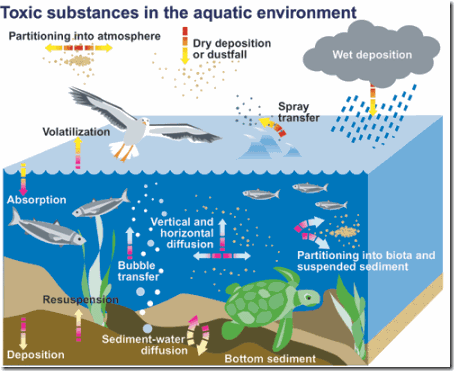
Wet and dry deposition of airborne pollutants is a sometimes overlooked, yet significant, source of chemical pollution of the oceans. For example, sulfur dioxide from a factory smokestack begins as air pollution. The polluted air mixes with atmospheric moisture to produce airborne sulfuric acid that falls on water and land as acid rain. This deposition can change the chemistry and ecology of an aquatic ecosystem. The major transport of PCBs to the ocean, for example, occurs through airborne deposition.
Industrial chemicals can adversely affect the growth, reproduction, and development of many marine animals. Pollutants are appearing not only in the Pacific, Atlantic, and Indian Oceans and their marginal seas, but also in the more remote and once-pristine polar oceans. An array of contaminants have been found in the flesh of fish and marine mammals in polar regions. In addition to the environmental and ecological issues, there is growing concern over the potential human health impacts in aboriginal communities whose residents depend on fish and marine mammals for daily sustenance.
A major public health concern is the safety of seafood as it relates to the chemical pollution of waters used for commercial and recreational fishing and mariculture . Heavy metals (e.g., copper, lead, mercury, and arsenic) can reach high levels inside marine animals, and then be passed along as seafood for humans. A well-known case of human poisoning occurred in Japan, where one industry dumped mercury compounds into Minimata Bay from 1932 to 1968. Methyl mercury that accumulated in fish and other animals was passed along to humans who consumed them. Over 3,000 human victims and an unknown number of animals succumbed to what became known as "Minimata Disease", a devastating illness that affects the central nervous system.
Monitoring by fisheries, environmental, and public health agencies can prevent or minimize cases of human illness caused by chemical contaminants in seafood. Some shellfish-producing areas off the U.S. coasts have been either permanently closed or declared indefinitely off-limits by health officials as a result of this type of pollution. A large percentage of U.S. fish and shellfish consumption advisories are due to abnormally high concentrations of chemical contaminants in seafood.
Regulatory Controls
The 1890 River and Harbors Act prohibited any obstruction to the navigation of U.S. Waters, and hence regulated the discharge of dredged material into inland and coastal waters. By weight, dredged material comprises 95 percent of all ocean disposal on a global basis. Its regulation (administered by the U.S. Army Corps of Engineers) increasingly is being accomplished in concert with broader concerns, including ecological integrity and other public interests.
In 1972, the U.S. Congress passed the Marine Protection, Research, and Sanctuaries Act (Ocean Dumping Act) and the Federal Water Pollution Control Act Amendments (Clean Water Act) that, among other goals, prohibited the disposal of waste materials into the ocean, and regulated the discharge of wastes through pipelines into the ocean. The Ocean Dumping Act requires the federal review of all proposed operations involving the transportation of waste materials for the purpose of ocean dumping, and calls for an assessment of the potential environmental and human health impacts. The U.S. Army Corps of Engineers and U.S. Environmental Protection Agency implement the permit programs associated with these laws.
In the United States, ocean dumping of industrial wastes is prohibited. Yet the vastness of the open sea provides a haven for illegal dumping.
The Ocean Dumping Ban Act of 1988 significantly amended portions of the 1972 Ocean Dumping Act, and banned ocean dumping of municipal sewage sludge and industrial wastes (with limited exceptions) by phased target dates. The disposal of sewage sludge in waters off New York City was a major motivation for its enactment. Ocean disposal of sewage sludge and industrial waste was totally banned after 1991. Narrow exceptions were created for certain U.S. Army Corps of Engineers dredge materials that occasionally are deposited offshore. Dredging is necessary to maintain navigation routes for trade and national defense. Consequently, allowable ocean dumping in the United States since 1991 has essentially been limited to dredge material and fish wastes.
Two international conferences in 1972—the UN Conference on the Human Environment, and the Intergovernmental Conference on the Convention on the Dumping of Wastes at Sea—were the result of international recognition of the need to regulate ocean disposal from land-based sources on a global basis. These conferences resulted in an international treaty, the Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter (also known as the London Convention).
Another treaty addressing the issue of wastes disposed from vessels was adopted in 1973. The International Convention for the Prevention of Pollution from Ships (or MARPOL) calls for signatory nations to enforce bans on dumping oil and noxious liquids into the ocean from ships, but the disposal of hazardous substances, sewage, and plastics remains optional.
As per the U.S. regulations, the dumping of industrial wastes, radioactive wastes, warfare agents (chemical or biological), sewage, and incineration at sea are directly prohibited. Moreover, the ocean disposal of other waste materials containing greater than trace amounts of certain chemicals is strictly prohibited. Allowed under strictly regulated conditions are the ocean disposal of relatively uncontaminated dredged material (harbor sediments), geologic material, and some fish waste; burial at sea; and ship disposal.
In 2000, the U.S. Congress enacted the Beaches Environmental Assessment and Coastal Health Act (BEACH Act) to reduce the risk of disease to users of the nation's coastal and Great Lakes waters. Funds are being made available for states and tribes to establish monitoring programs for disease-causing microbes, and to notify the public when monitoring indicates and public health hazard.
EUTROPHICATION:
Introduction
Corals require the cleanest water quality of any coastal ecosystem, and suffer if it deteriorates. One crucial aspect of water quality is the concentration of nutrients in the water. Nutrients are essential elements needed for the growth of all forms of life, and when they are inadequate, organisms are unable to grow well, no matter how much other food is available. In coastal waters two nutrients, nitrogen and phosphorus, are typically present in such low concentrations that they prevent full growth. In the remote open ocean iron and other trace metals can also be scarce, but this is rarely the case in coastal waters due to abundance of these elements on suspended clays.
Coral reefs have evolved in the lowest nutrient environment in the world, the tropical ocean, where plants often consume all available nitrogen and phosphorus, at which point new growth is limited to rates at which these elements are provided by decomposition of dead organisms. Although there is abundant nitrogen and phosphorus in the deep sea it cannot reach surface layers where bright light promotes the growth of plants and animals because a thick layer of warm waters floating on top of deep cold nutrient-rich water prevents its being mixed upward to the surface.
Tiny increases in nutrients above the near zero level are probably beneficial to corals, but it takes only very small increases for the net effect to turn negative. This is not because high nutrients harm corals directly, but because corals are quickly overgrown by much faster growing algae which need higher nutrient levels than corals. Only very little excess nutrients are needed to turn healthy coral reefs into waving fields of algae which smother and kill corals. This phenomenon is called eutrophication. Eutrophication takes place in all ecosystems and is responsible for green scummy layers of algae covering ponds into which sewage and manure flows. Coral reefs go eutrophic at the lowest level of nutrients of any aquatic ecosystem: nutrient levels which would be regarded as very low in any other marine or freshwater habitat will kill coral reefs. This web site has papers discussing these problems, and their solutions, in more detail.
Major sources of excessive nutrients include sewage, livestock manures, agricultural fertilizers, soils eroding away after deforestation, and upwelling of deep ocean waters. Where nutrient inputs are episodic, for example where there are strongly seasonal rivers, tourist sewage, or upwelling inputs reefs may be eutrophic part of the year only. Where nutrients continue to increase, coral will be killed. Due to the large increase of nutrients released into coastal waters from sewage discharged directly into the ocean or delivered via rivers and ground waters, the reefs off all coastal areas which are densely populated or developed for tourism are already eutrophic or quickly turning so. This can happen very rapidly, and in only a few years healthy reefs can be turned into coral graveyards. Probably no coral reef country is free of this problem, not even the smallest.
Only in recent years have we have learned just how low nutrients must be to maintain healthy coral reefs. The limits were found independently by two researchers working on opposite sides of the globe, who were not aware of each other's work. By looking at the relative amounts of corals and algae along nutrient gradients from intense land-based sources, namely agricultural fertilizers in Australia and bird droppings on a mangrove island in Belize, Peter Bell and Brian Lapointe independently determined exactly the same limit for acceptable nutrient concentrations. Biologically available nitrogen (nitrate plus ammonia) needs to be below 1.0 micromole per liter (less than 0.014 parts per million of nitrogen), and biologically available phosphorus (orthophosphate plus dissolved organic phosphorus) needs to be below 0.1 micromole per liter (less than 0.003 parts per million of phosphorus). In addition concentrations of chlorophyll (in the microscopic plants called phytoplankton) needs to be below 0.5 parts per billion.
These values are all regarded as extremely low levels, almost undetectable, in coastal waters of temperate and cold zones. For years researchers measured concentrations in this range but thought that values were too low to possibly cause problems to reefs. This was wrong because they used irrelevant standards for acceptable nutrient levels. It is essential that appropriate water quality standards be applied in coral reef ecosystems if they are to be protected against eutrophication. These standards must be below the levels given above. In general, where water quality standards have been applied for tropical waters, they are often based on uncritical adoption of nutrient standards from North America and Europe that are irrelevant to the tropics because cold ecosystems are normally exposed to much higher nutrient levels. Many nutrient water quality standards available are related to human health and are even more worthless for coral reefs because humans can drink water with nutrient levels hundreds of times higher than coral reefs can stand.